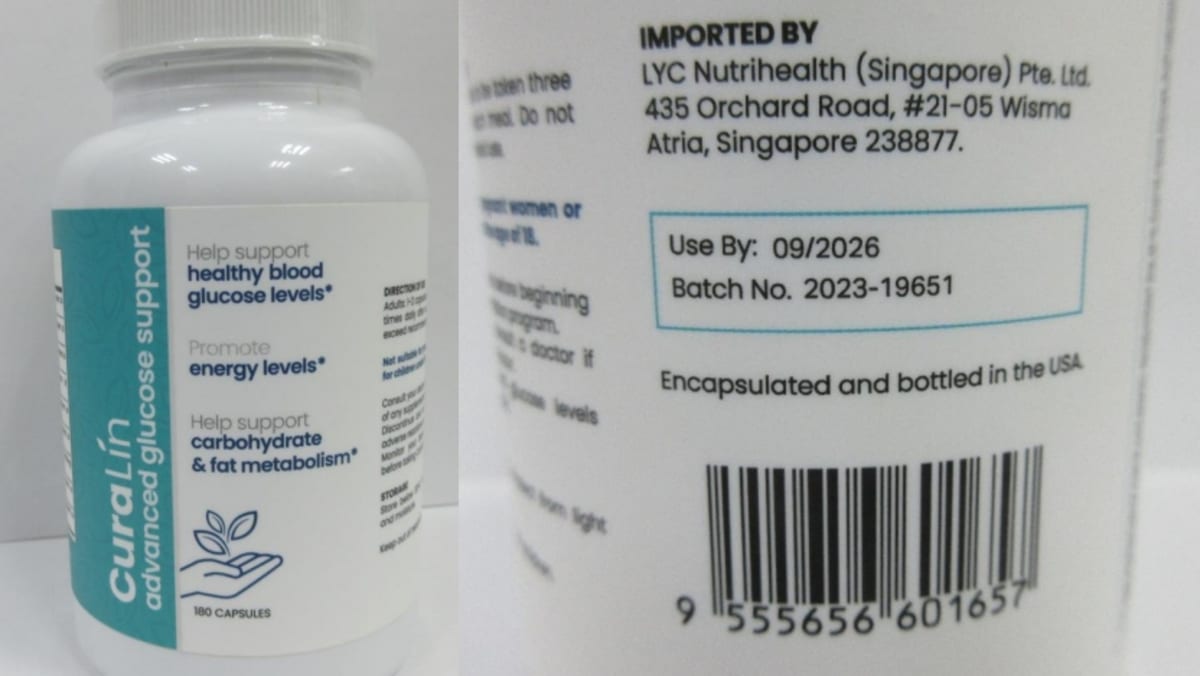
SINGAPORE: The Health Sciences Authority (HSA) on Monday (May 5) recalled a blood glucose supplement after it was found to contain undisclosed prescription-only antidiabetic medicines.
In a press release, HSA warned the public not to consume “CuraLin advanced glucose support”, as the product, which was labelled to contain traditional herbs, was tested by the authority during routine checks to contain glibenclamide and metformin.
Glibenclamide and metformin are prescription-only medicines used to treat diabetes mellitus and should only be taken under medical supervision.
HSA has directed LYC Nutrihealth, which imported the product in two batches, to stop all sales and recall both batches.
It added that the supplement was brought in from the United States and supplied locally with the following batch numbers: Batch 2023-19650 and Batch 2023-19651, both of which expire in September 2026.
The recall is ongoing.
HSA said that the inappropriate use of glibenclamide or metformin without medical supervision may cause low blood glucose levels, which can lead to seizures and coma.
“Other adverse effects include nausea, vomiting, diarrhoea, and cholestatic jaundice, a condition where bile flow is blocked or slowed down.”
Metformin can also cause lactic acidosis – a dangerous build-up of acid in blood – that can be life-threatening, HSA added.
Diabetic patients who take the supplement together with their prescribed antidiabetic medicines are also at risk of overdose due to the additive effects of these medicines, said the authority.
CuraLin advanced glucose support was marketed to “help support healthy blood glucose levels”, “promote energy levels”, and “help support carbohydrate and fat metabolism”.
It was sold by various sellers and on local e-commerce platforms, including Shopee and Lazada.
“HSA has worked with the local e-commerce platform administrators to ensure that listings of the affected product were removed,” it said, adding that all sellers and suppliers must stop selling the product immediately.
Consumers are advised to stop consuming the product immediately.
Those who feel unwell after taking the product, especially if they have diabetes or have been concurrently taking other prescribed antidiabetic medicines, should consult their doctor.
“There may be other batches or variants of ‘CuraLin advanced glucose support’ being sold in the market. If in doubt, consumers should not buy or consume these products,” said HSA.
For returns of the affected batches of the product, consumers should contact the retailer or seller from whom they bought the product.
HSA said it will not hesitate to take stern enforcement actions against anyone who sells and supplies products found to be adulterated with potent ingredients.
Sellers and suppliers are liable to prosecution and, if convicted, may be jailed for up to two years and/or fined up to S$10,000 (US$12,900).